Page 18 - English isu 43
P. 18
Mohamed Rashwan
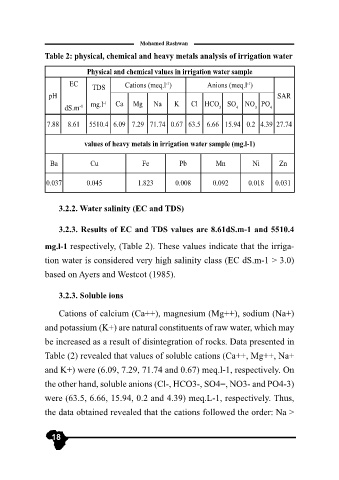
Table 2: physical, chemical and heavy metals analysis of irrigation water
Physical and chemical values in irrigation water sample
EC TDS Cations (meq.l ) Anions (meq.l )
-1
-1
pH SAR
dS.m -1 mg.l -1 Ca Mg Na K Cl HCO 3 SO 4 NO 3 PO 4
7.88 8.61 5510.4 6.09 7.29 71.74 0.67 63.5 6.66 15.94 0.2 4.39 27.74
values of heavy metals in irrigation water sample (mg.l-1)
Ba Cu Fe Pb Mn Ni Zn
0.037 0.045 1.823 0.008 0.092 0.018 0.031
3.2.2. Water salinity (EC and TDS)
3.2.3. Results of EC and TDS values are 8.61dS.m-1 and 5510.4
mg.l-1 respectively, (Table 2). These values indicate that the irriga-
tion water is considered very high salinity class (EC dS.m-1 > 3.0)
based on Ayers and Westcot (1985).
3.2.3. Soluble ions
Cations of calcium (Ca++), magnesium (Mg++), sodium (Na+)
and potassium (K+) are natural constituents of raw water, which may
be increased as a result of disintegration of rocks. Data presented in
Table (2) revealed that values of soluble cations (Ca++, Mg++, Na+
and K+) were (6.09, 7.29, 71.74 and 0.67) meq.l-1, respectively. On
the other hand, soluble anions (Cl-, HCO3-, SO4=, NO3- and PO4-3)
were (63.5, 6.66, 15.94, 0.2 and 4.39) meq.L-1, respectively. Thus,
the data obtained revealed that the cations followed the order: Na >
18